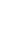In Vivo PK
Greentech is proud of offering a broad spectrum of pharmacokinetic (PK) services, including in vitro ADME, in vivo pharmacokinetic studies and bioanalysis. In vivo PK studies are essential to characterize PK properties of drug candidates in animals. Our capabilities include an experienced pharmacokinetic team, AAALAC-accredited animal facilities and state-of-the-art equipment.
Species: non-human primates, dogs, rabbits, guinea pigs, rats/mice.
Administration Routes: oral, sublingual, topical, subcutaneous (SQ), intraperitoneal (IP), intramuscular (IM), intravenous (IV) rapid bolus, IV infusion, etc.
Biological Matrices: blood, plasma, bile, urine, feces and tissues
Mass Balance: excretion kinetics in urine and bile, tissue distribution
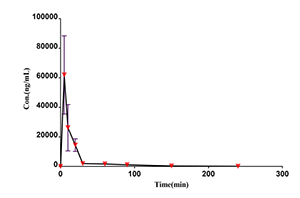
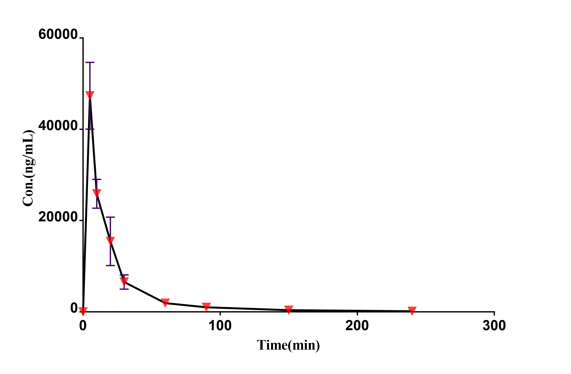
Case Study
Drug concentration-time curve
 | 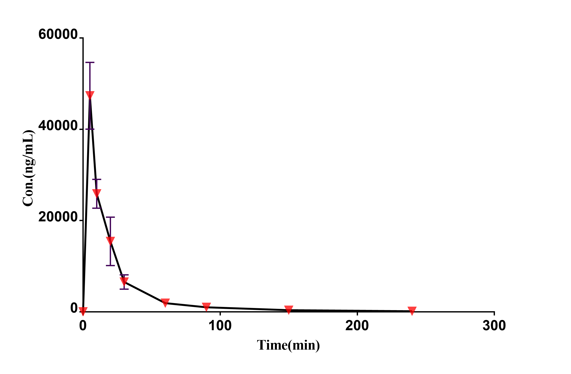 |
|
| Low-dose group | Medium-dose group | High-dose group |
Inquiries
Request a quote now, or email us at BD@greentech-bio.com to inquire about our services or obtain a quote for your project.