In Vitro ADME
The evaluation of pharmacological properties including absorption, distribution, metabolism, and excretion (ADME) is critical for screening novel drug candidates. Greentech has rich experience in pharmacokinetics, including in vitro ADME, in vivo PK and small molecule bioanalysis. At lead generation and optimization stages, we provide in vitro ADME screening and validated assays.
ADME Screening Assays
Permeability tests: PAMPA,Caco-2,MDR1-MDCK
Metabolic stability tests: liver microsomes from human and preclinical species
CYP450 enzyme inhibition tests: single or mixed substrates, estimation of percentage inhibition or IC50
Plasma protein binding tests: plasma isolated from human and preclinical species
Whole blood/plasma distribution tests: multiple species
Validated Assays
CYP450 enzyme inhibition tests: 8 CYP subtypes, estimation of IC50, Ki, Kinact, reversible/irreversible inhibition, time-dependent inhibition (TDI) of CYP
CYP450 enzyme induction tests: frozen human hepatocytes, estimation of enzyme activity and mRNA level
CYP phenotype analysis: multiple species
Plasma protein binding tests: equilibrium dialysis, ultrafiltration and ultracentrifugation
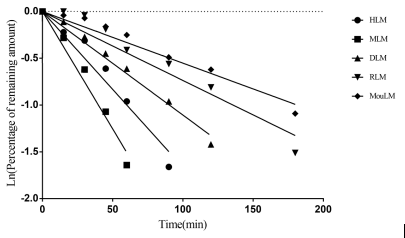
Case Study
Inquiries
Request a quote now, or email us at BD@greentech-bio.com to inquire about our services or obtain a quote for your project.