Models of Myocardial Ischemia and Infarction
Greentech Bioscience offers left anterior descending (LAD) coronary artery ligation models, coronary occlusion models, and isoproterenol (ISO)-induced myocardial infarction (MI) models for efficacy testing of novel treatments.
Our Animal Models of Myocardial Ischemia and Infarction
1. LAD Ligation Induced Models of Myocardial Ischemia and Infarction
Induction: left anterior descending (LAD) coronary artery ligation
Species: rats, pigs, dogs, non-human primates (NHPs)
Disease characteristics: mainly causing ischemia located in the anterior wall of the left ventricle
Advantages and disadvantages: LAD ligation is commonly used for establishing acute myocardial ischemia and infarction models, characterized by simple operations, optional occlusion site and controllable infarction range. The surgery with a high mortality rate has a high demand for technicians with excellent surgical skills and rich experience. However, LAD ligation models hardly mimic coronary atherosclerosis-induced myocardial infarction in human due to the lack of elevated lipid levels and coronary atherosclerotic lesions.
2. Coronary Occlusion Models
Induction: introduction of embolic agents into coronary artery by using Digital Subtraction Angiography (DSA) technique, resulting in LAD occlusion
Species: pigs, dogs, non-human primates (NHPs)
Advantages: animal models of coronary artery occlusion by using DSA technique are characterized by small surgical trauma, stable human disease characteristics, and flexible choice on which coronary artery to occlude. The balloon occlusion method allows for assessment of reperfusion injury after myocardial infarction.
3. ISO-Induced Rat Model of Myocardial Infarction
Induction: ISO injection
Species: rats
Disease characteristics: ISO induced MI model well mimicks vasoconstriction process, mainly resulting in diffuse myocardial injuries, instead of focal ischemic myocardial damage.
Clinical Assessment
Ultrasonic cardiogram: left ventricular end-diastolic diameter (LVEDD), left ventricular end-systolic diameter (LVESD), left ventricular end-diastolic volume (LVEDV), left ventricular end-systolic volume (LVESV), left ventricular posterior wall thickness (LPwT), ejection fraction (EF), cardiac output (CO), FS, SV, chamber dimensions and wall thickness, pulmonary artery pressure, etc.
Invasive hemodynamic monitoring: central venous pressure (CVP), right atrial pressure (RAP), right ventricular pressure (RVP), pulmonary artery pressure (PAP), pulmonary capillary wedge pressure (PCWP), cardiac output (CO)
12-lead electrocardiogram
Myocardial enzymogram and biochemical examination
Histopathology
Basic Service
Body weight, food intake, excretion
Blood and tissue collection
Optional Endpoint
Imaging examinations such as MRI
Coronary angiography (for large animals)
Case Study
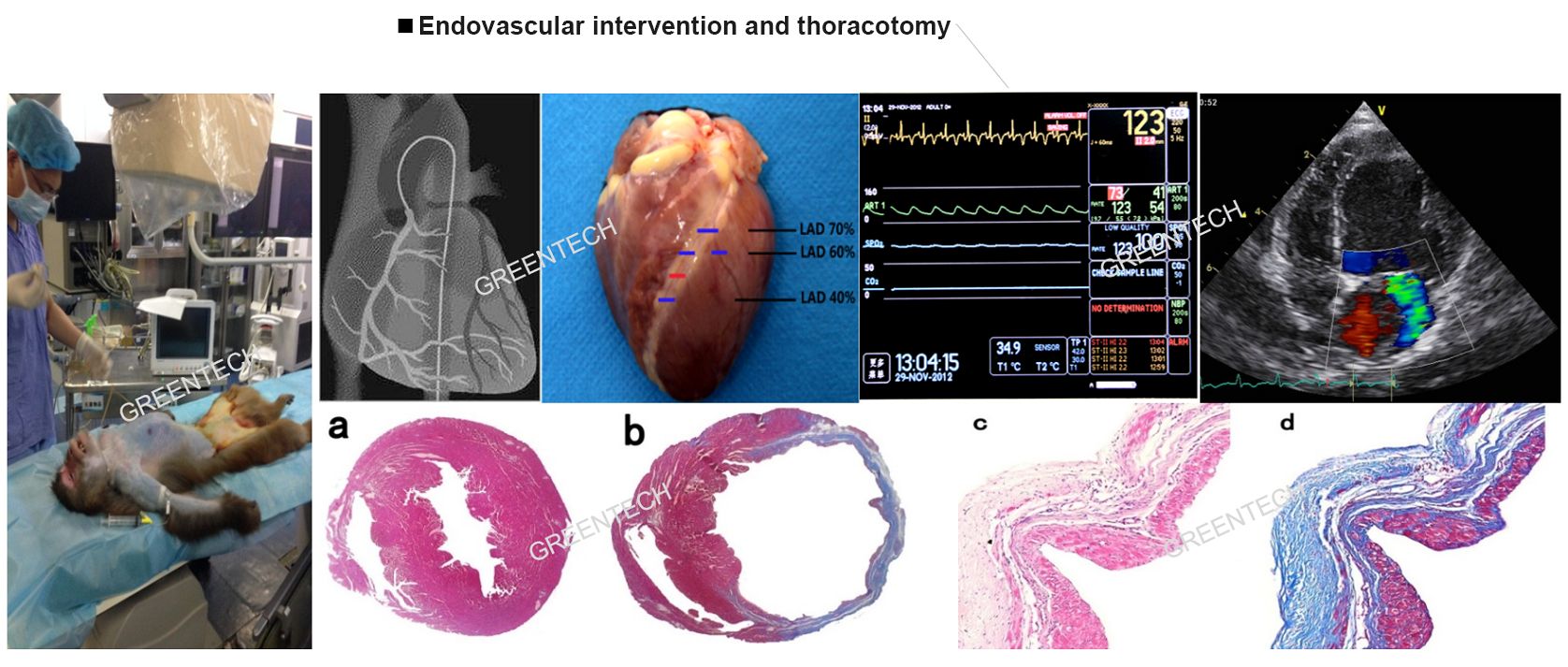
Figure 1. Endovascular intervention and thoracotomy.
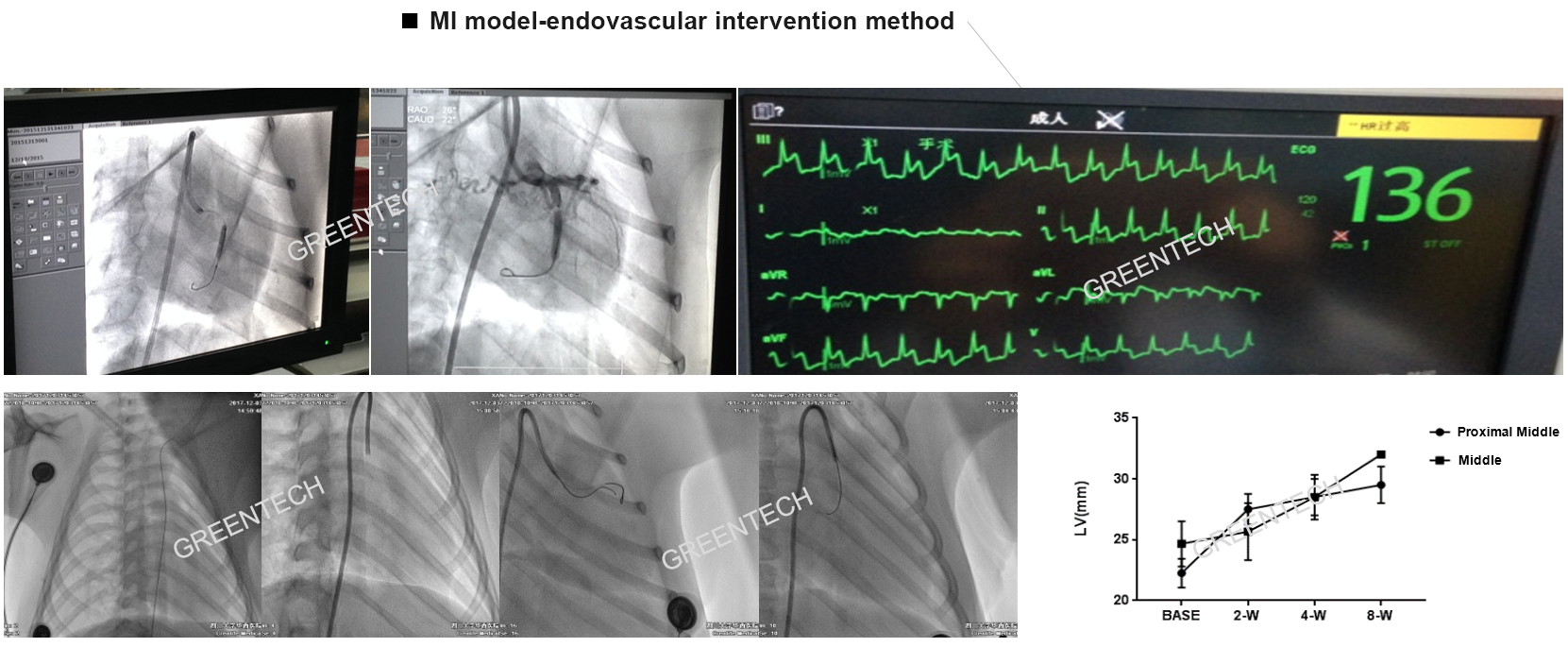
Figure 2. Myocardial infarction model-endovascular intervention method; Balloon occlusion VS gelatin sponge.
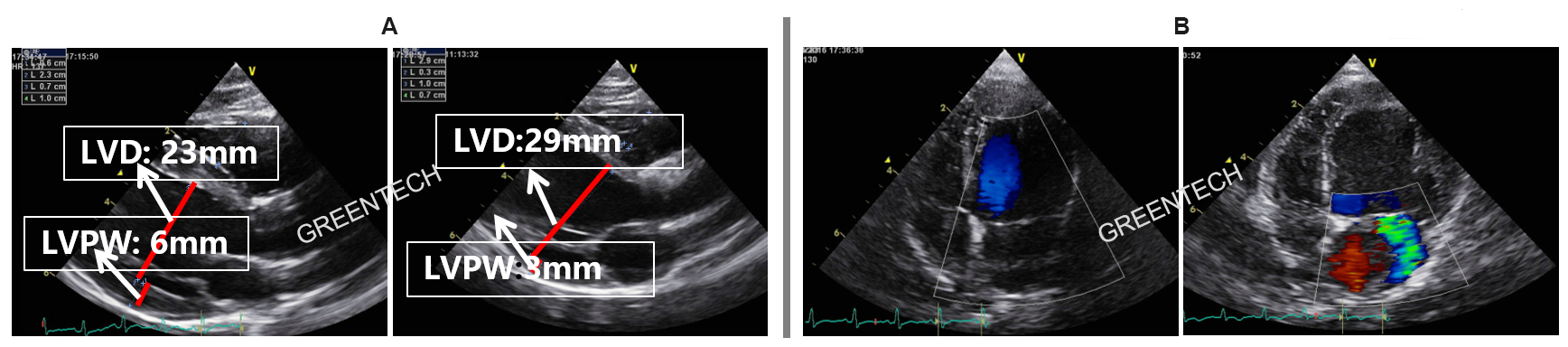
Figure 3. Myocardial ischemia-induced ventricular remodeling: left ventricular enlargement and left ventricular wall thickening; Ischemic mitral regurgitation: stable midsystolic mitral regurgitation until 8 weeks post surgery.
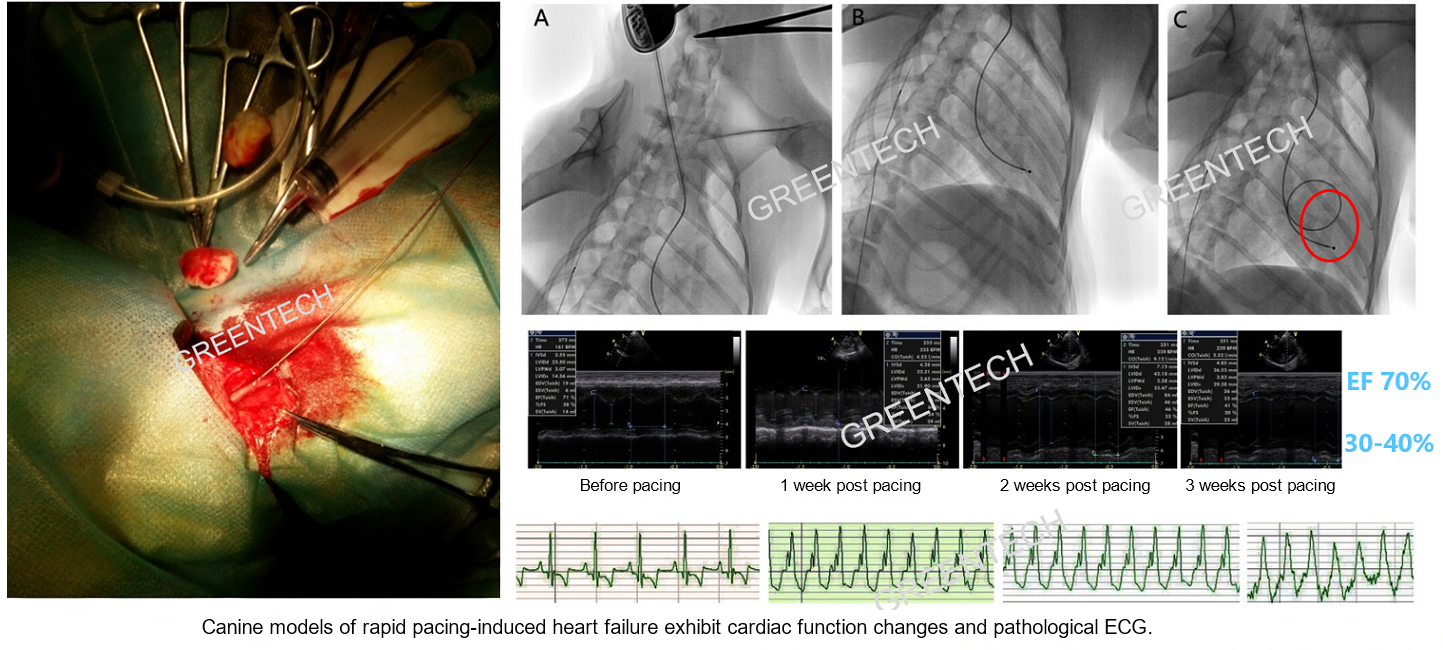
Figure 4. Canine models of rapid pacing-induced heart failure exhibit cardiac function changes and pathological ECG.
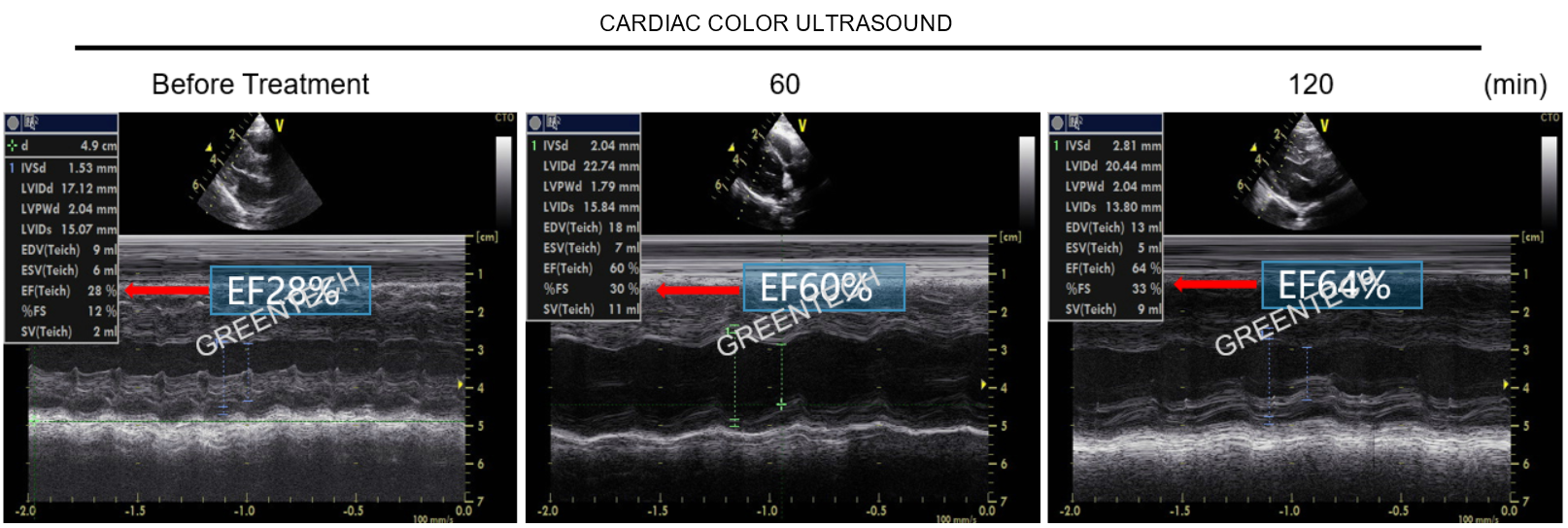
Figure 5. Evaluation of * drug in NHP models of rapid pacing-induced heart failure (color Doppler ultrasound examination) .
References
1. Milani-Nejad, N., & Janssen, P. M. L. (2014). Small and large animal models in cardiac contraction research: Advantages and disadvantages. Pharmacology & Therapeutics, 141(3), 235–249. doi:10.1016/j.pharmthera.2013.10.007
2. Dobrev, D., & Wehrens, X. H. T. (2018). Mouse Models of Cardiac Arrhythmias. Circulation Research, 123(3), 332–334. doi:10.1161/circresaha.118.313406
Inquiries
Request a quote now, or email us at BD@greentech-bio.com to inquire about our services or obtain a quote for your project.












