Small Molecule Bioanalysis
Bioanalytics is an essential tool for determining the concentration of biomarkers, drugs and their metabolites in various biological fluids. Bioanalytical method validation serves as a critical step ensuring that quantitative data demonstrate selectivity, accuracy and stability. Greentech provides high quality small molecule bioanalysis services, including development and validation of LC-MS/MS methods, method transfer, and quantitative sample analysis in human and animal matrices (plasma, tissues, urine and feces), contributing to preclinical and clinical development of small molecule drugs.
Our Small Molecule Bioanalysis Services
Bioanalytical method development/validation/transfer
Validation content:
Selectivity
Carry-over
Calibration curve and lower limit of quantification
Accuracy and precision
Dilution integrity
Matrix effect
Stability
Quantitative analysis of biomarkers, drugs and toxins in biological samples
Case Study
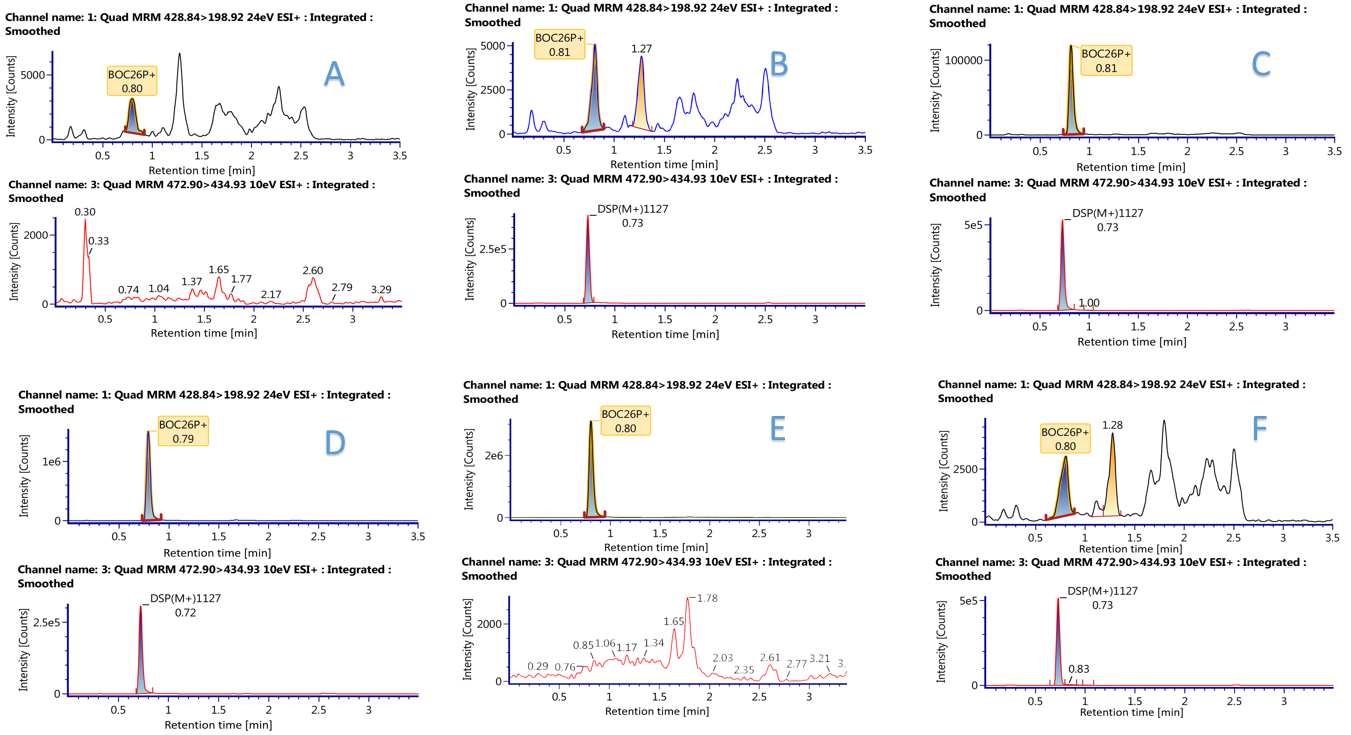
Figure 1. Typical validation of selectivity.
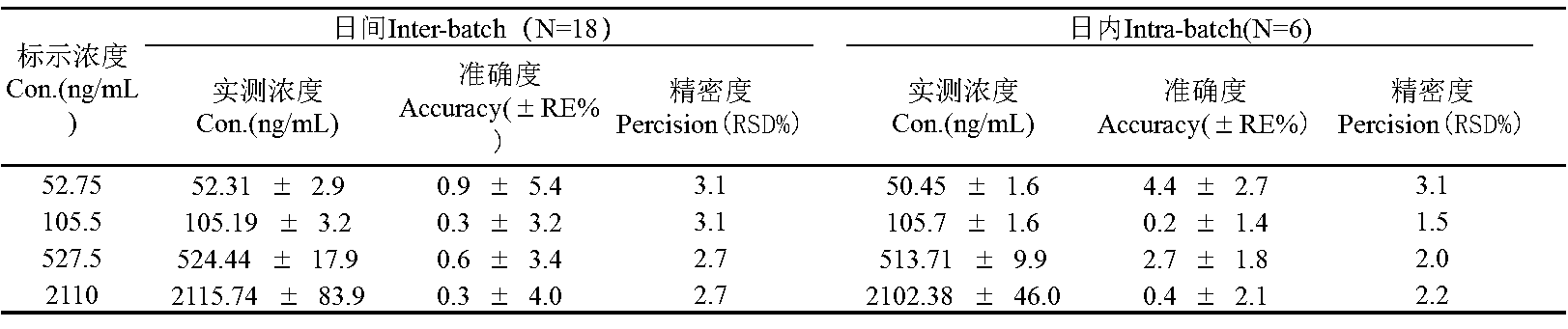
Figure 2. Accuracy and precision values of the HPLC-MS/MS method for the determination of a compound in rat plasma.
Inquiries
Request a quote now, or email us at BD@greentech-bio.com to inquire about our services or obtain a quote for your project.